 Group of Dr. Traube - Faculty for Chemistry and Pharmacy
Group of Dr. Traube - Faculty for Chemistry and Pharmacy
 Group of Dr. Traube - Faculty for Chemistry and Pharmacy
Group of Dr. Traube - Faculty for Chemistry and Pharmacy
Our goal is to image chemical reactions on solid surfaces with atomic resolution. Heterogeneous catalysis, a cornerstone of modern industrial chemistry, is based on such reactions. To image the atomic processes, we develop special variants of scanning tunneling microscopy (STM). We support this work with other surface methods, e.g., photoemission spectroscopy and low-energy electron microscopy.
In our research we aim for a better understanding of elementary surface processes as well as the underlying mechanisms of industrially relevant chemical reactions.
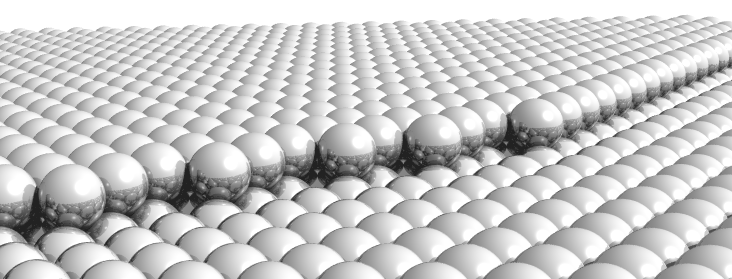
Figure: Model of a close-packed cobalt surface with a monoatomic step. The reactions of the cobalt-catalyzed Fischer-Tropsch synthesis take place on such surfaces.