X-Ray Photoelectron Spectroscopy (XPS)
In XPS a sample is illuminated by soft X-rays leading to the emission of photoelectrons. The kinetic energy of the photoelectrons is measured, mostly by a hemispherical electron analyzer. The electrons originating from atomic core orbitals are specific for the element, and in the typically used energy range the electrons mainly come from the surface. These properties make XPS the most important spectroscopy for chemical analysis of surfaces. We use it to analyze cobalt and silver surfaces in our catalysis projects. We use a monochromatized X-ray source allowing us to discriminate adsorbed particles in different chemical states by their chemical shifts.
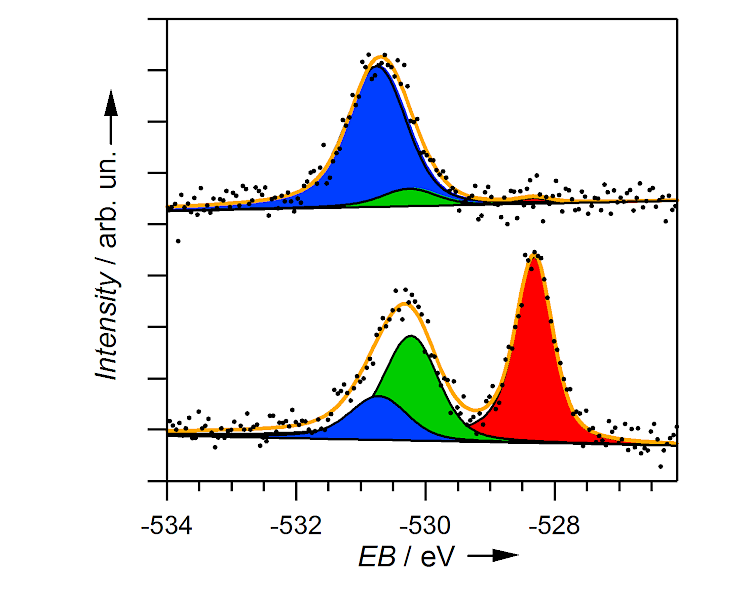
Figure: XP spectra of the O 1s peak of oxygen adsorbed on an Ag(111) surface. The spectra show three different oxygen species that may play a role in the silver-catalyzed synthesis of ethylene epoxide. Reproduced with permission from "High-pressure scanning tunneling microscopy of a silver surface during catalytic formation of ethylene oxide", S. Böcklein, S. Günther and J. Wintterlin, Angew. Chem. Int. Ed. 2013, 125, 5628. Copyright Wiley-VCH Verlag GmbH & Co. KGaA.