Fischer-Tropsch Synthesis on Cobalt Model Catalysts
In the industrial Fischer-Tropsch synthesis a mixture of hydrogen and carbon monoxide, so-called "syngas", reacts to give hydrocarbons. The products are mainly linear paraffins and olefins:
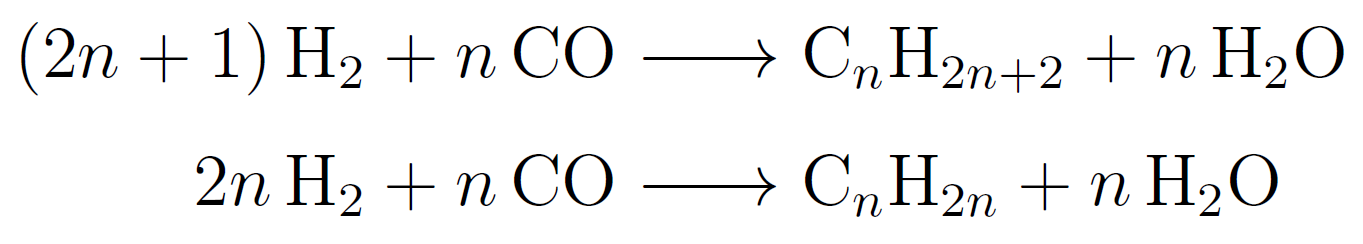
The process is expected to play a key role in the production of liquid fuels in the future and may also be used for energy storage. The catalyst in the modern Fischer-Tropsch synthesis consists of small metallic cobalt particles on oxide supports. The reaction mechanism has been studied for decades but, like with most other catalytic reactions, essential questions have remained open. In particular, the structure and chemical state of the active cobalt surface are unclear. To obtain an atomic view of the operating catalyst we use a high-pressure STM under temperature and pressure conditions close to those of the industrial process. For the cobalt catalyst we use cobalt single crystal models. Recently, we were able to identify monoatomic steps as active sites of the Fischer-Tropsch synthesis. Operando STM studies showed that catalytic activity scales with step density of the Co catalyst.
Recent publications:
- "Kinetics of the Fischer–Tropsch Synthesis on Cobalt Single Crystals under Scanning Tunneling Microscopy Control",
S. Kläger, K. Golder and J. Wintterlin, ACS Catal. 2023, 13, 14685–14698. - "In Situ/Operando STM of the Fischer–Tropsch Synthesis on a Co(10-115) Surface–A Study to Bridge the Materials Gap between Single-Crystal Models and Supported Catalysts",
K. Golder and J. Wintterlin, ACS Catal. 2022, 12, 7199–7209. - "The active sites of a working Fischer–Tropsch catalyst revealed by operando scanning tunnelling microscopy",
B. Böller, K. Durner and J. Wintterlin, Nat. Cat. 2019, 2, 1027–1034.